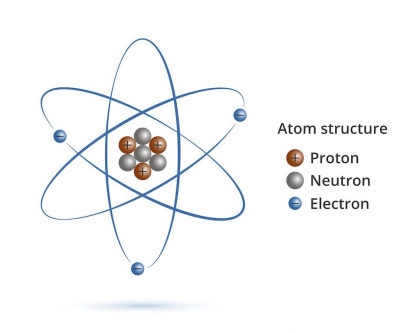
Everything is made up of atoms which are the smallest parts of an element still possessing the chemical properties of that element. It is difficult to realize how small atoms are. They have a diameter of about one-hundred-millionth of a centimeter. At one time scientists believed atoms were little spheres that could not be broken, but we know that atoms are composed of other particles which are even smaller. Each atom is like a miniature solar system: at the centre it has a nucleus which consists of protons and neutrons around which electrons revolve.
The atom consists almost entirely of empty space and its entire size is that of the orbit of its outer electron, which revolves at extremely high velocity, forms an impenetrable shield. A propeller going round very fast will give us an idea of an electron. The electron seems to be at every point of its orbit at the same time because it goes round the nucleus so fast. That is why we say the atom consists mostly of empty space. The spherical shield formed by the revolving electrons prevents the emptiness between their orbits and the nucleus from being filled in normal circumstances.
The nucleus and the electrons each have a diameter of about one-tenth of a millionth part of millionth part of one centimeter. Nearly all the mass of the atom is contained within the nucleus. The electrons are very light compared with the protons and the neutrons which are 1,837 times heavier than the electrons.
Electrons have a negative charge and they are fixed to the atom and cannot break away from their orbits through centrifugal force because protons have an equivalent positive charge and the two balance each other. Neutrons have no electrical charge.
Picture Credit : Google
