MOLECULES
An element is a substance made of only one kind of atom. Atoms join, or bond, to other atoms of the same element, or to atoms of other elements. They do this by sharing or exchanging electrons. In many cases, the bonded atoms form groups called molecules. When atoms of one element combine with atoms of other elements, they form compounds.
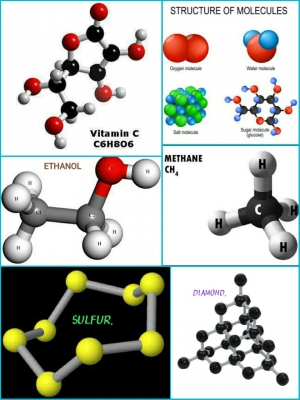
- ETHANOL (C2H5OH) Ethanol is a compound with molecules containing two carbon (C) atoms, six hydrogen (H) atoms, and one oxygen (O) atom. At room temperature, it is a colourless liquid used as a disinfectant, preservative, and the alcohol in drinks.
- OXYGEN (O2) Some elements can bond in several ways to form different substances. Atoms of the element oxygen (O) bond together in pairs to form molecules of oxygen gas. High in the atmosphere, however, oxygen atoms also bond in threes to form the gas ozone.
- VITAMIN C (C6H8O6) Like ethanol, vitamin C (ascorbic acid) contains carbon (C), hydrogen (H), and oxygen (O) atoms. However, because the molecule contains different quantities of the elements arranged in a different way it forms an entirely different substance – a compound that is solid at room temperature.
- METHANE (CH4) Molecules of the gas methane contain four hydrogen (H) atoms bonded to a carbon (C) atom. Compounds of carbon and hydrogen are called hydrocarbons. They include fuels, such as methane, oil, and coal, and artificial materials, such as polystyrene.
- SALT (NaCl) Table salt, or sodium chloride, is made of equal numbers of sodium (Na) and chlorine (CI) atoms. Salt does not form as small individual molecules containing one atom each of sodium and chlorine. Instead, many atoms of the two elements link to form a rigid lattice called a crystal.
- WATER (H2O) When two atoms of the gas hydrogen (H) bond with one atom of the gas oxygen (O), they form molecules of liquid water. Water is the most common compound on Earth and is essential for life.
- SULFUR (S8) Eight atoms of sulfur (S) bond together in a ring to form a sulfur molecule. Most non-metal elements are gases at room temperature, but sulfur is a brittle, yellow solid.
- AMMONIA (NH3) The colourless gas ammonia is made up of one nitrogen (N) atom bonded to three hydrogen (H) atoms. Plants get the nitrogen they need to grow from ammonia and other nitrogen compounds within the soil.
- DIAMOND (C) A diamond can be thought of as a single giant molecule. It is formed from atoms of carbon (C) bonded in a strong crystal lattice. However, if carbon atoms join in flat sheets of hexagonal tiles, they form graphite, a brittle substance used to make pencil leads.
Picture Credit : Google



