Was cobalt widely used in Chinese pottery?
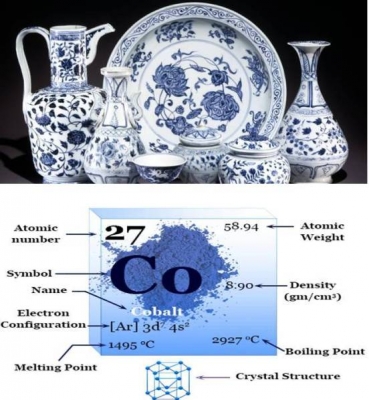
In ancient China, cobalt salts were used to decorate pottery with brilliant blue designs. Cobalt was discovered as an element in the 1730s. It was isolated by George Brandt, a Swedish chemist in 1735 for the first time.
The atomic number of cobalt is 27. In its pure form, cobalt is silvery-blue and brittle. Its properties are similar to iron and nickel. It can be made magnetic like iron. Because of this property, some high-powered magnets are made from alloys of cobalt and aluminium or nickel. Its man-made radioactive isotope, Cobalt-60, is used in cancer treatments.
Cobalt is also used in alloys to build parts of aircraft engines and in manufacturing alloys with corrosion/wear-resistant properties. It is widely used in making batteries and in electroplating. Cobalt is also an essential element for many living creatures. It is a component of vitamin B12.
In August 2014, a team of astrophysicists reported the presence of cobalt-56 in the supernova SN2014J which is an exploding star 11 million light-years from Earth!
Picture Credit : Google







































