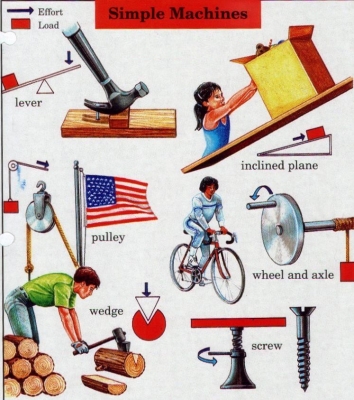
What simple machines make work easier?
MACHINES
Simple machines make it easier for people to perform tasks, such as lifting or pulling, which would be difficult to do with muscle strength alone. There are six types of simple machines: the wheel, the screw, the lever, the pulley, the inclined plane, and the wedge. These machines change a force into a bigger or smaller force, or alter the direction in which a force acts. The most basic tools, such as crowbars or spades, are simple machines.
- WHEEL (GEAR) Gears are toothed wheels that mesh and turn together, changing the strength, speed, or direction of a force. A force on the axle of a small gear driving a large gear will lead to a bigger turning force on the axle of the large gear.
- SCREW The spiral thread on a screw changes a turning force into a much stronger up or down force. The screw has to be turned many times to create just a small up or down movement.
- LEVER Most levers magnify the force applied to them, making it easier to move a load. A lever turns around a fixed point called a pivot. The further from the pivot the force is applied, the easier it is to move the load.
- PULLEY A pulley is a rope looped around a wheel to make a load easier to lift or move. The more ropes and wheels are used, the less force is needed to lift the load, but the further the rope has to be pulled.
- INCLINED PLANE This is a flat surface with ends at different heights. Moving an object up an inclined plane reduces the amount of force needed to lift it up, but increases the distance it has to travel.
- WEDGE This triangular object is used as a blade to split something or inserted under an object to lift it. As a downward force is exerted on the wedge, its widening shape produces a sideways force on the object.
- CART WHEELS These wheels allow the cart to move smoothly up the ramp. Unlike gear wheels, these are not classed as a machine, because they do not change the size of the force applied to them to help do something.
COMPOUND MACHINE A device that operates using a combination of simple machines, called a compound machine. Human force is applied only once - to turn the gear wheel. Each simple machine applies a force to the next machine until the tomato is sliced in two.
Picture Credit : Google