What is water and its importance?

WATER
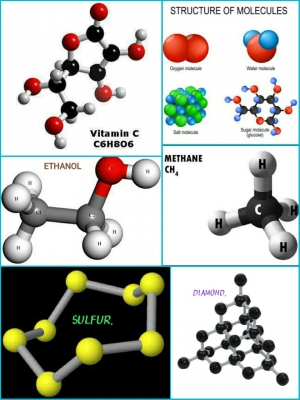
Water is a tasteless, odorless liquid. Although it appears to be colourless, in fact it is very pale blue. Each molecule of water is made up of two hydrogen atoms and one oxygen atom, giving it the chemical formula H2O. Water is Earth’s most common compound, found everywhere, from the oceans that cover 71 per cent of the planet to each cell of every living organism.
- Unlike most compounds, water can exist in all three states of matter, solid, liquid, and gas, within Earth’s normal range of temperatures.
- At sea level, water is liquid between 0 and 100°C (32-212°F), but below 0°C (32°F), it solidifies into ice, and above 100°C (212°F), it becomes gaseous water vapour.
- Unlike most other substances, water is denser when it is liquid than when it is solid - that is why ice floats on the top of water instead of sinking.
- When water freezes into ice, it expands by nine per cent of its volume with a force that can burst pipes and split rock.
- Earth is the only place in the Solar System where conditions allow water to exist in liquid form at the surface. Some liquid water exists under the surface of the moons of Jupiter and Saturn.
- Water is essential for life, so astronomers look for it when searching for life on other planets.
- The body of an average adult man contains more than 40 litres (70 pints) of water.
- You need about 2 litres (4 pints) of water every day to keep healthy.
- About 30 per cent of the world’s population do not have clean, safe running water at home.
- Water is not a resource that can be used up like oil. Water evaporates into the air, forms clouds, and falls back to Earth as rain. In areas of Earth that receive little rainfall, water can be a scarce resource.
Picture Credit : Google