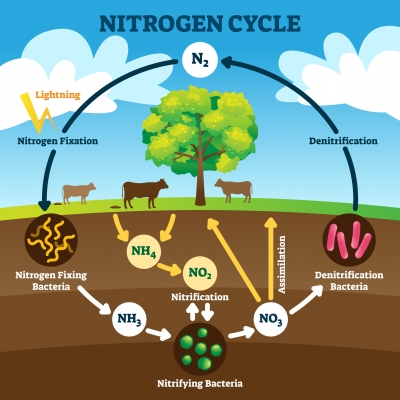
Our atmosphere is made up of 78% nitrogen. This element is essential for all living beings but we cannot directly take the nitrogen from the environment. We must absorb it through our food. The nitrogen cycle follows the circulation of nitrogen from the atmosphere to the soil, to animals and back. Nitrogen in the atmosphere falls to the earth through snow and rain. Once in the soil, the nitrogen combines with the hydrogen on the roots of the plants to form ammonia. This process is called Nitrogen fixation. Additional bacteria further combine this ammonia with oxygen in a process called Nitrification. At this point, the nitrogen is in a form called nitrite, which is further converted into nitrate by the bacteria. Plants can absorb nitrogen in this state through a process called assimilation and the rest is utilised by the bacteria. The remainder is released back into the atmosphere through the process of denitrification.
Nitrogen Cycle Explained – Stages of Nitrogen Cycle
Process of the Nitrogen Cycle consists of the following steps – Nitrogen fixation, Nitrification, Assimilation, Ammonification and Denitrification. These processes take place in several stages and are explained below:
Nitrogen Fixation Process
It is the initial step of the nitrogen cycle. Here, Atmospheric nitrogen (N2) which is primarily available in an inert form, is converted into the usable form -ammonia (NH3).
During the process of Nitrogen fixation, the inert form of nitrogen gas is deposited into soils from the atmosphere and surface waters, mainly through precipitation.
The entire process of Nitrogen fixation is completed by symbiotic bacteria, which are known as Diazotrophs. Azotobacter and Rhizobium also have a major role in this process. These bacteria consist of a nitrogenase enzyme, which has the capability to combine gaseous nitrogen with hydrogen to form ammonia.
Nitrogen fixation can occur either by atmospheric fixation- which involves lightening, or industrial fixation by manufacturing ammonia under high temperature and pressure conditions. This can also be fixed through man-made processes, primarily industrial processes that create ammonia and nitrogen-rich fertilisers.
Assimilation
Primary producers – plants take in the nitrogen compounds from the soil with the help of their roots, which are available in the form of ammonia, nitrite ions, nitrate ions or ammonium ions and are used in the formation of the plant and animal proteins. This way, it enters the food web when the primary consumers eat the plants.
Ammonification
When plants or animals die, the nitrogen present in the organic matter is released back into the soil. The decomposers, namely bacteria or fungi present in the soil, convert the organic matter back into ammonium. This process of decomposition produces ammonia, which is further used for other biological processes.
Denitrification
Denitrification is the process in which the nitrogen compounds make their way back into the atmosphere by converting nitrate (NO3-) into gaseous nitrogen (N). This process of the nitrogen cycle is the final stage and occurs in the absence of oxygen. Denitrification is carried out by the denitrifying bacterial species- Clostridium and Pseudomonas, which will process nitrate to gain oxygen and gives out free nitrogen gas as a byproduct.
Conclusion
Nitrogen is abundant in the atmosphere, but it is unusable to plants or animals unless it is converted into nitrogen compounds.
Nitrogen-fixing bacteria play a crucial role in fixing atmospheric nitrogen into nitrogen compounds that can be used by plants.
The plants absorb the usable nitrogen compounds from the soil through their roots. Then, these nitrogen compounds are used for the production of proteins and other compounds in the plant cell.
Animals assimilate nitrogen by consuming these plants or other animals that contain nitrogen. Humans consume proteins from these plants and animals. The nitrogen then assimilates into our body system.
During the final stages of the nitrogen cycle, bacteria and fungi help decompose organic matter, where the nitrogenous compounds get dissolved into the soil which is again used by the plants.
Some bacteria then convert these nitrogenous compounds in the soil and turn it into nitrogen gas. Eventually, it goes back to the atmosphere.
These sets of processes repeat continuously and thus maintain the percentage of nitrogen in the atmosphere.
Credit : BYJU’S
Picture Credit : Google